News
Pulse Technologies Announces Successful Biocompatibility Results For Novel HSR™ Platinum-Iridium Electrode Surface Treatment Technology
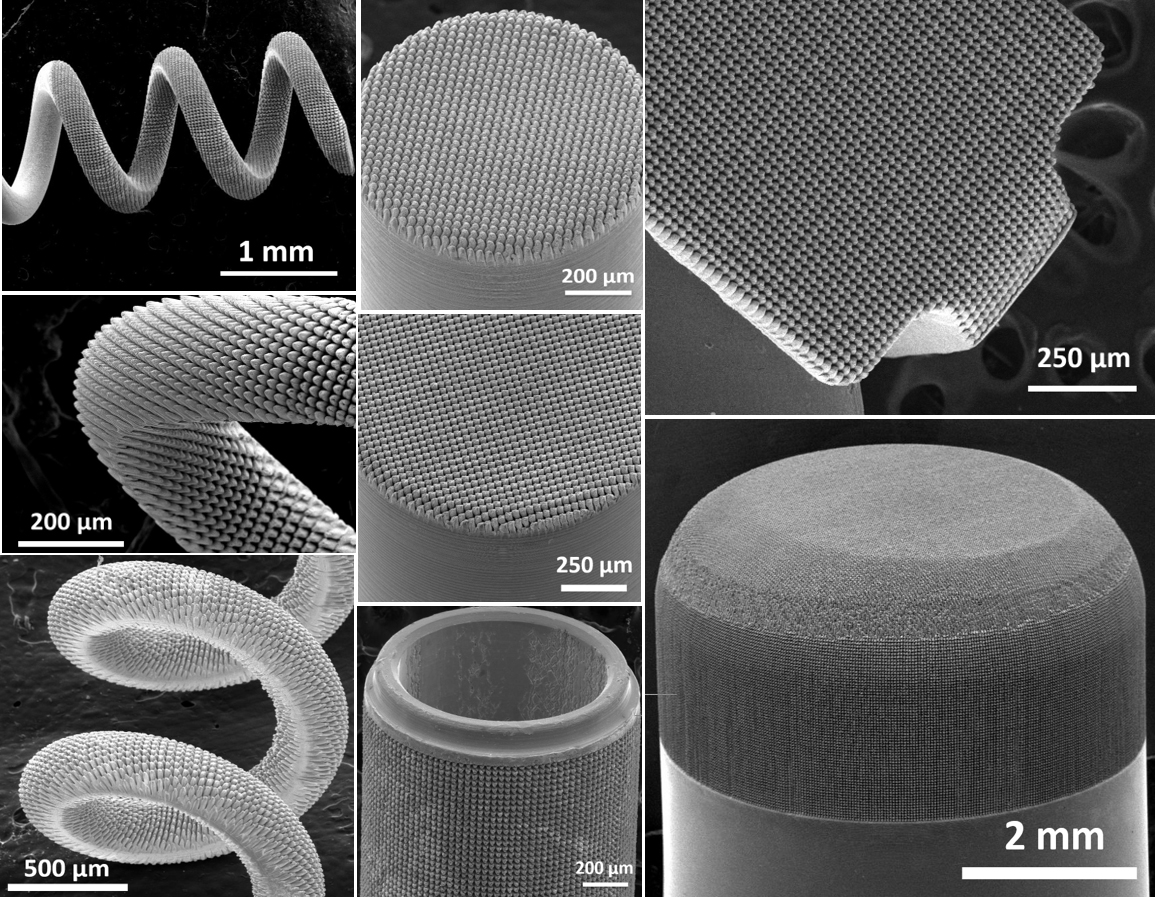
Biocompatibility studies were conducted by an independent, certified lab based in the US in accordance with FDA and ISO standards.
Pulse Technologies Inc., a US-based contract manufacturer with 25+ years of medical device manufacturing experience, is pleased to announce successful biocompatibility test results for their novel Hierarchical Surface Restructuring (HSR™) technology.
Biocompatibility of HSR™ electrodes was evaluated by performing comprehensive cytotoxicity, sensitization and irritation testing. The laboratory where these measurements were conducted is accredited in accordance with the recognized International standard ISO/IEC 17025:2017.
HSR™, A Ground-Breaking Technology for The Medical Device Industry
This patented surface treatment technology was developed to enhance implantable electrodes’ performance. Medical device companies can partner with Pulse Technologies to leverage HSR™ to improve device performance, longevity and effectiveness. The use of HSR™ also gives way to opportunities for electrode miniaturization, a growing need in the medical device industry.
HSR™ has applications in:
- Neuromodulation
- Cardiac Rhythm management
- Electrophysiology mapping catheters
- Cochlear implants
The next generation of medical devices in the above-mentioned areas can all benefit from the use of Pulse’s HSR™ technology. This technology is applicable on both electrodes and assembled leads. It is easy to use, requires no masking and there is no coating-like overspray.
HSR™ Benefits for Medical Device Companies
The benefits include:
- Improved electrode longevity – no cracking or delamination when implanted
- Tuning capability for desired electrochemical performance
- Engineered surface topography
- Unprecedented improvement in charge storage capacity and capacitance
- Substantial reduction in impedance
HSR™ Meets All Biocompatibility Requirements
Three biocompatibility studies were conducted on HSR™ treated Pt10Ir electrodes as outlined below:
- Elution Cytotoxicity Test: this is an in-vitro procedure which allows for a qualitative assessment of cytotoxicity to determine the biological reactivity of mammalian cell cultures following incubation with the extracts of test articles.
- Intracutaneous (Intradermal) Reactivity Test: this study was conducted to assess the potential of HSR™ electrodes to cause irritation following a single intradermal injection of specific extracts prepared from the test articles.
- Skin Sensitization Test: this test was conducted to determine the potential of the HSR™ electrodes to elicit contact dermal allergenicity.
HSR™ developed by Pulse Technologies successfully met all the biocompatibility requirements as outlined by the above tests. The studies conducted were all reviewed in accordance with the provisions of the FDA Good Laboratory Practice Regulations (21 CFR, Part 58).
At Pulse Technologies, we offer:
· 25+ years of medical device manufacturing experience.
· Industry-leading in-house metrology and testing capabilities
· 70,000 sq. ft. facility.
· 100+ machine centers.
· Data conveniently formatted for DHFs/DHRs, and much more.
Want to learn more? Get in touch: sales@pulsetechnologies.com.
Contract Manufacturing
Advanced Technology
Capabilities
© 2021 Pulse Technologies, Inc. All rights reserved.
 Careers
Careers Contact
Contact







